Canfield Crucible Sets
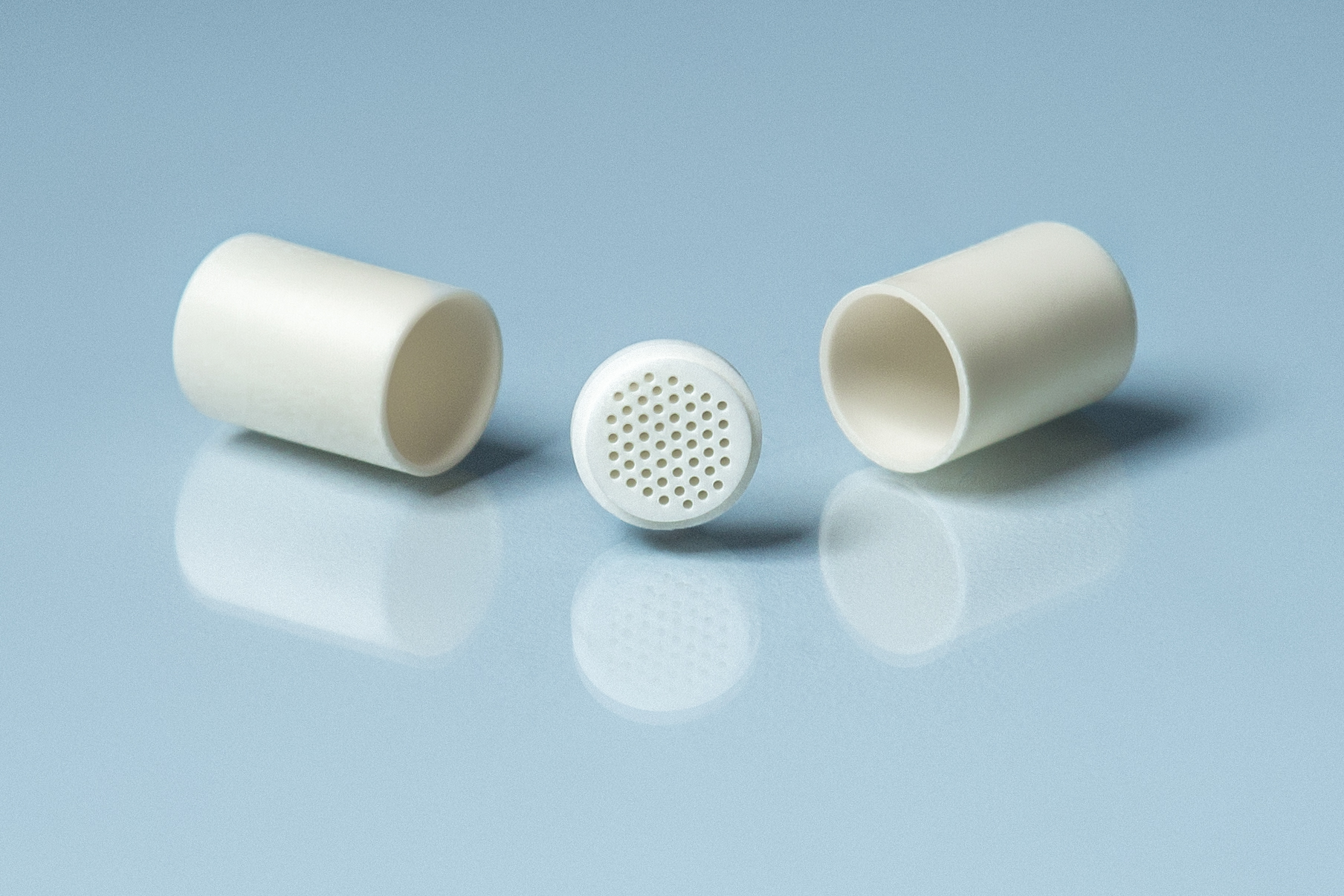
Types of Canfield Crucible Sets
ACP-CCS Canfield Crucible Set, (2) CYC002 2ml capacity cylindrical crucibles, 13mm OD x 25mm high with (1) 25 hole strainer
ACP-CCS-5 Canfield Crucible set, (2) 5ml capacity cylindrical crucibles, 18mm OD x 25mm high with (1) 50 hole strainer
ACP-CCS-1.7 Canfield Crucible set, (2) 1.7ml capacity cylindrical crucibles, 10,5mm OD x 25mm high with (1) 25 hole strainer
What Are Canfield Crucible Sets (CCS)
Canfield Crucible Sets (CCS) are two cylindrical crucibles separated by a frit-disc that allows for separation of liquid from solid phase. The frit-disc has a milled shoulder on each side to make what can be a step-taper that allows for simple and effective co-axial alignment of the three-part assembly. The CCS is often used as the containment vessel for solution growth of single crystals. The CCS can be sealed into a morphous silica tubes or even tantalum tubes. The precise dimensions of the set can be chosen to fit within specific tubing. Separation of crystalline phase from remaining liquid is often accomplished with the aid of a laboratory centrifuge.
Clean Separation for Crystal Growth
Canfield Crucible Sets are designed to eliminate contamination during the separation of solid and liquid phases. The frit disc design ensures clean decanting, allowing researchers to reuse residual solutions for further growth experiments without silica fiber interference.
Precision Alignment and Assembly
The step tapered frit disc shoulders provide simple, effective coaxial alignment of the crucible assembly. This precision engineering ensures reliable performance in high temperature crystal growth processes, reducing errors and improving reproducibility.
Versatility In Research Applications
CCS can be sealed into silica or tantalum tubes, making them adaptable for a wide range of laboratory setups. Their compatibility with centrifuge assisted separation further enhances their utility in advanced materials science and intermetallic compound research.
Cost Effective and Reliable Design
Compared to traditional methods, Canfield Crucible Sets offer a more affordable, contamination free solution for crystal growth. Their durable alumina construction and reusable design make them a long term investment for laboratories focused on high temperature synthesis.
FAQ
What Makes Canfield Crucible Sets Unique?
They use a frit disc design that enables clean separation of liquid and solid phases without contamination.
Can Residual Solutions Be Reused With CCS?
Yes. The clean separation allows decanted liquids to be reused for further crystal growth experiments.
What Materials Are Used in CCS Construction?
The crucibles are made of alumina, with stainless steel bushings and graphite seals for durability.
In What Research Fields Are CCS Commonly Used?
They are widely used in materials science, chemistry, and semiconductor research for single crystal growth.
For in-depth details please refer to a full article in Philosophical Magazine, Volume 96, Issue 1, pages 84-92 (ww.tandfonline.com/doi/full/10.1080/14786435.2015.1122248)
Paul C. Canfield, Tai Kong, Udhara S. Kaluarachchi & Na Hyun Jo
Abstract: Solution growth of single crystals from high temperature solutions often involves the separation of residual solution from the grown crystals. For many growths of intermetallic compounds, this separation has historically been achieved with the use of plugs of silica wool. Whereas this is generally efficient in a mechanical sense, it leads to a significant contamination of the decanted liquid with silica fibers. In this paper, we present a simple design for frit-disc alumina crucible sets that has made their use in the growth single crystals from high temperature solutions both simple and affordable. An alumina frit-disc allows for the clean separation of the residual liquid from the solid phase. This allows for the reuse of the decanted liquid, either for further growth of the same phase, or for subsequent growth of other, related phases. In this paper, we provide examples of the growth of isotopically substituted TbCd and icosahedral i-RCd quasicrystals, as well as the separation of (i) the closely related and phases and (ii) and ...
